Temperature Mapping
Temperature mapping is a crucial step in the qualification process
of temperature-controlled units. It involves systematically
measuring and mapping the temperature distribution within the unit
to ensure uniformity and accuracy across the entire storage or
processing area. Here are the general steps involved in temperature
mapping:
-
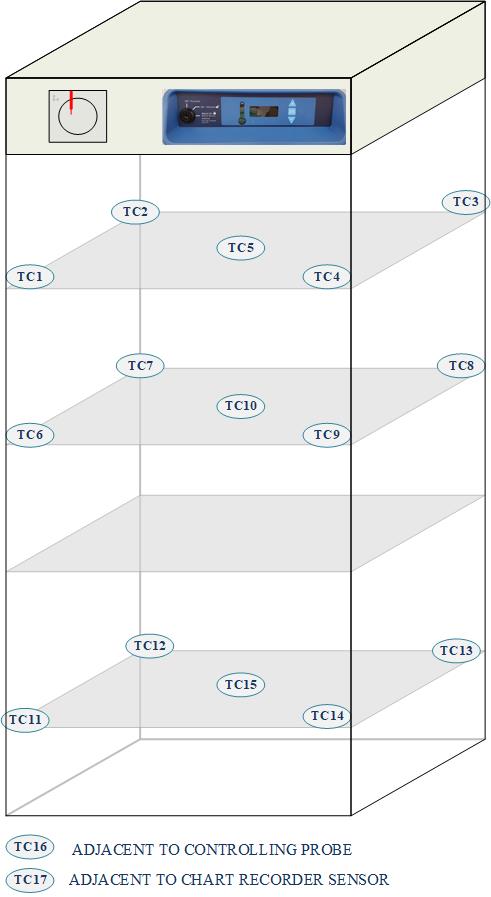
Define the Mapping Study: Determine the scope and objectives of the temperature mapping study. Consider the size and layout of the temperature-controlled unit, the temperature range of interest, and any specific requirements or regulations.
-
Select Sensors: Choose appropriate temperature sensors or data loggers to accurately measure and record temperature. Select sensors with suitable accuracy, precision, and response time. Consider the number and placement of sensors to ensure representative coverage of the entire unit.
-
Sensor Placement: Position the temperature sensors at predetermined locations within the temperature-controlled unit. Distribute the sensors evenly to capture temperature variations throughout the space. Place sensors in areas that are most critical or challenging, such as near heat sources or at the furthest points from the temperature control source.
-
Study Conditions: Stabilize the temperature-controlled unit by allowing sufficient time for temperature equilibrium. Ensure that the unit is empty or contains a representative load of product or placeholders to simulate normal operating conditions. Close the unit to minimize air flow interference.
-
Data Collection: Start the data collection process by recording the temperature measurements from the sensors at defined time intervals. The duration of the data collection period may vary depending on the requirements and industry guidelines. Capture temperature data continuously or at regular intervals to create a comprehensive temperature profile.
-
Data Analysis: Analyze the collected temperature data to assess the temperature distribution and uniformity within the unit. Calculate statistical parameters such as mean, minimum, maximum, standard deviation, and temperature differentials to evaluate compliance with acceptance criteria. Identify any hot spots, cold spots, or areas of concern.
-
Report Generation: Document the temperature mapping study findings in a comprehensive report. Include information about the study methodology, sensor placement, data collection process, analysis results, and any deviations or observations. Summarize the temperature distribution and uniformity, highlighting any non-compliant areas.
-
Corrective Actions: If any non-compliance or deviations are identified during the temperature mapping study, take appropriate corrective actions to address the issues. This may involve adjusting the temperature control settings, optimizing the unit's airflow, or implementing other measures to improve temperature uniformity.
Temperature mapping provides critical information about the performance and uniformity of temperature-controlled units. It helps identify areas of concern, optimize storage or processing conditions, and ensure the integrity of temperature-sensitive products.
The Kaye Validator system is typically used for temperature mapping studies, thermal uniformity testing, and stability studies in controlled environments. It helps verify that the temperature and humidity conditions within these environments meet the required specifications. The system can perform both static and dynamic validation, allowing for comprehensive assessment of temperature and humidity variations during different operational conditions.
The Kaye Validator System Components:
-
Kaye Validator Hardware: The hardware consists of temperature and humidity sensors, a data logger, and a display unit. The sensors are placed at various locations within the controlled environment to measure and record temperature and humidity values. The data logger collects and stores the sensor data, while the display unit provides real-time monitoring and analysis of the measurements.
-
Kaye Validator Software: The software is used to configure the validation parameters, manage the data logger, and analyze the collected data. It allows users to set up validation profiles, define acceptance criteria, and generate comprehensive reports. The software also provides advanced data analysis tools, including statistical calculations, graphical representations, and customizable data views.
-
Calibration Accessories: To ensure the accuracy and reliability of the measurement system, calibration accessories are used. These include calibration standards, reference sensors, and calibration certificates. Regular calibration of the Kaye Validator system is essential to maintain traceability and compliance with regulatory requirements.
When using the Kaye Validator system, it is important to follow good validation practices and adhere to relevant regulatory guidelines, such as those outlined by regulatory authorities like the FDA and international standards like ISO 17025. Proper installation and placement of sensors, accurate calibration, and appropriate data analysis are critical for obtaining reliable validation results.